Our Focus
Large scale genetic screens have identified many significant genes that contribute to neuropsychiatric and neurodevelopmental diseases, like bipolar disorder and autism spectrum disorders. What we lack is an understanding of how these genetic alterations impact protein function and ultimately brain activity and behavior. Ankyrins, encoded by the ANK1-3 genes, have been implicated in a wide range of brain diseases. Ankyrins are intracellular scaffolding proteins that have critical functions in the formation of plasma membrane domains in polarized cells throughout the body, especially in the brain. Our laboratory is interested in ankyrins, their protein partners, and how dysfunction of these protein complexes contributes to disease.
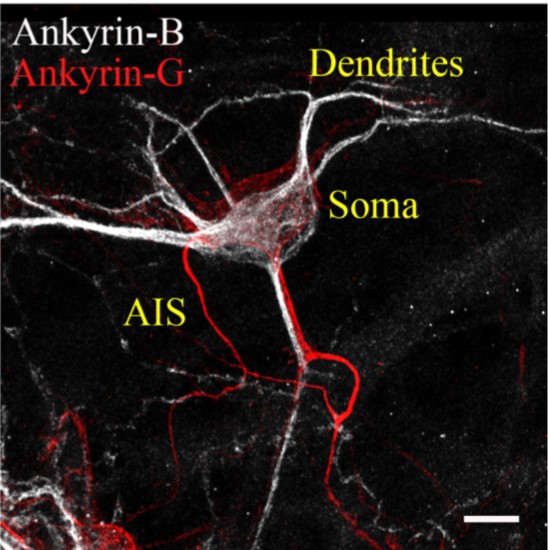
Ankyrin-G in Bipolar Disorder
Multiple genetic studies have shown that the ANK3 gene is highly associated with bipolar disorder (BD). The ANK3 gene encodes ankyrin-G, a scaffolding protein involved in the formation of the axon initial segment (AIS), nodes of Ranvier, and GABAergic synapses. We generated a Ank3 mutant mouse that has striking reductions in inhibitory neurotransmission. Importantly, alterations in inhibitory signaling causes an excitatory / inhibitory imbalance that has also been observed in BD patients. We recently identified a BD family carrying the same ANK3 variant, highlighting the potential impact of ankyrin-dependent inhibitory signaling. Our team is investigating how common BD therapeutics affect ankyrin-dependent inhibitory signaling in Ank3 mutant mouse models.
Ankyrin-B in Autism Spectrum Disorders
Multiple genetic studies have shown that the ANK2 gene is highly associated with autism spectrum disorder (ASD). ANK2 encodes ankyrin-B, which has been found to localize in the dendrites, however its role there remains poorly understood. Recent data show that Nav1.2, encoded by SCN2A, is critical for normal dendritic function. SCN2A is the top gene associated with ASD. We hypothesize that Nav1.2 is recruited to, and maintained at, the dendrites by ankyrin-B. We aim to understand how these two genes, ANK2 and SCN2A, are linked at the molecular level and how dysfunction of this pathway contributes to disease.
Mechanisms Underlying Ankyrin Targeting in Polarized Cells
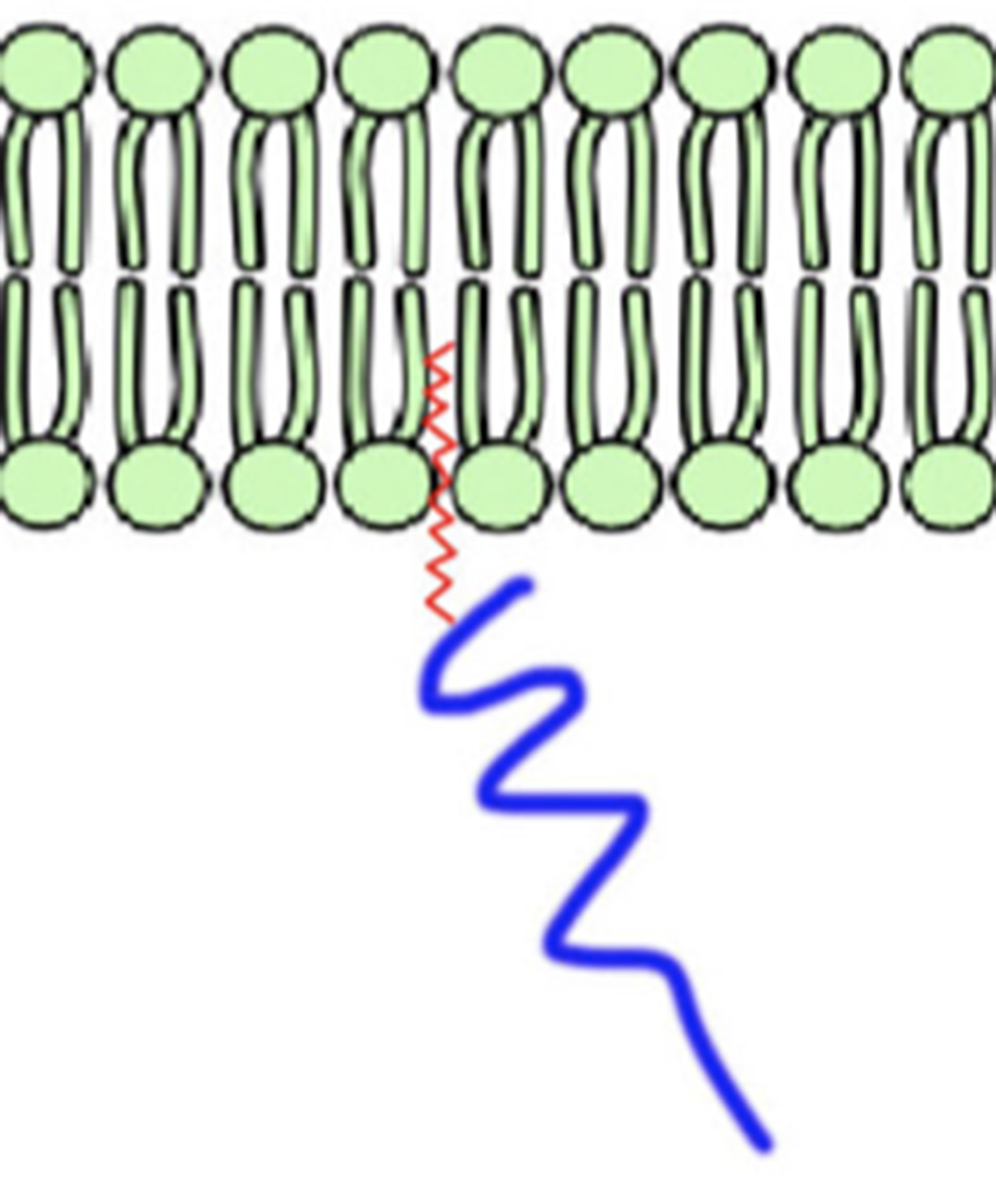
Ankyrins are important for localizing their binding partners at specific membrane domains, but very little is known about how ankyrins themselves reach these membrane sites. Previous work has demonstrated that ankyrin-G relies on the post-translational modification, S-palmitoylation, which is the addition of a 16-carbon fatty acid to cysteine residues of proteins, to localize specifically at the lateral membrane of epithelial cells and at the AIS of neurons. Our laboratory is investigating whether ankyrin-B also undergoes S-palmitoylation to regulate its localization and downstream functions. These studies help us understand how alterations in these localization mechanisms may contribute to ankyrin-associated disease such as BD or ASD.